
pH
Remember middle school science class? Don't worry - it'll all come back to you. Maybe.
Citrus trees like a pH of about 6.5 as this is where all the nutrients required for their everyday life are available.
Citrus can typically tolerate anywhere from 6-7.5 pH. Outside of this range, certain minerals become “locked up” or unavailable due to their chemical properties. This means that if the pH is too high or too low, no matter how much fertilizer you throw at your plant, those specific nutrients cannot be used!
-
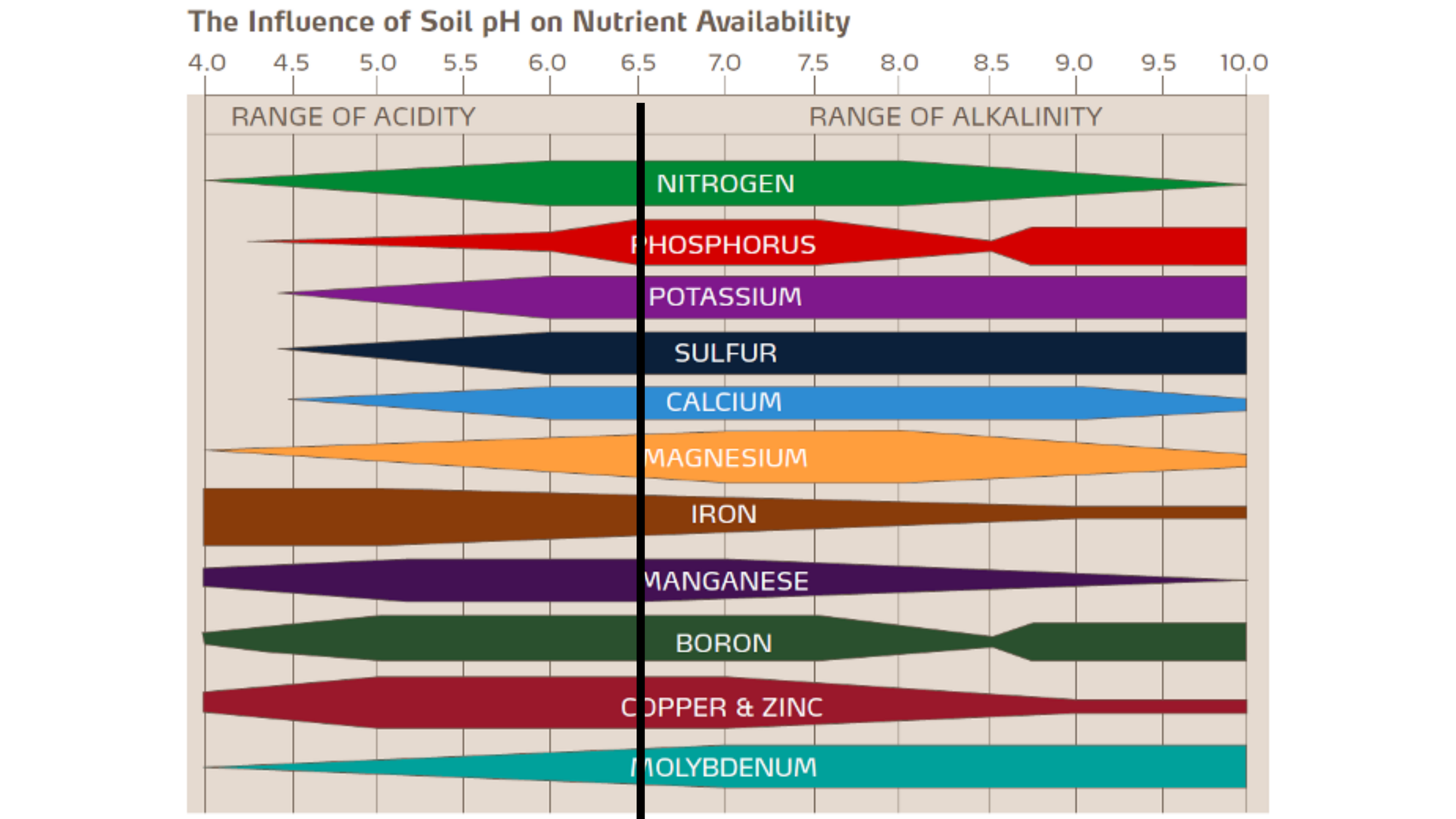
Illustration by Yara.us; Citrus Agronomic Principles.
-
As a citrus tree approaches a higher pH, the availability of iron, copper, zinc, and manganese becomes very slim. The black line on the graph represents the optimal 6.5 pH where all the necessary nutrients are perfectly accessible to the plant. If you fertilize according to the instructions and are still seeing nutrient deficiencies, check the pH of the water you’re using!
Correcting pH
Are you worried the pH of your water might be outside the range your citrus tree prefers?
Our first recommendation is to head to your local garden store and pick up a pH pen (they are relatively low-priced). Calibrate it according to instructions and test your water.
If the pH is too high (the most likely scenario), you can add a little bit of vinegar or lemon juice to your water before you water your citrus tree.
If your pH is too low (fairly unlikely, especially in Wisconsin), you can usually find a "pH up" solution in the aquarium section of your local pet store.
Remember to add pH adjustments in increments. It doesn't take very much additive to make a huge difference in pH!
If you don't want to worry about the pH of your tap water, there are other solutions as well.
- Rainwater has the perfect pH for citrus trees (plus lots of essential nutrients!). While your tree is outside, it will love that natural water. If you're able to collect rainwater (or snow) during the winter time, your tree will thank you.
- Deionized water typically works well as it lacks minerals that influence pH.
More information
-
Citrus Tree Care 101
Time to go back to the basics? -
Controlling Pests
See how to control common pests for citrus trees. -
Common Issues
Yellow leaves? Losing leaves? We can help!
Questions?
Subscribe to our emails
Be the first to know about new collections and exclusive offers.

